Swine Nutrition Guide: Feed Additives
Feed additives -- Feed additives are compounds that may elicit a response independent of contributions to the pig’s energy, amino acid, mineral, and(or) vitamin requirements. Typically, these feed additives are added to pig diets in small amounts. In addition, certain nutrients, such as copper and zinc, have been added at pharmacological concentrations (i.e., at high levels the nutrient acts as a drug-see section on nutraceuticals).
How are feed additives regulated?
The distribution of all animal feeds entering interstate commerce is regulated by the FDA (Food and Drug Administration). In addition, the FDA monitors the amounts of drugs or feed additives used in the manufacturing of medicated feeds. Specific state laws and regulations may also exist regarding the distribution of feeds and the production of medicated feeds.
How does the FDA describe drug categories used in medicated feeds?
The program that describes the classification of drugs used in medicated animal feeds is commonly known as “Second Generation.” This regulatory scheme divides drugs into two major categories:
Category I
Drugs that require no withdrawal at the lowest approved continuous use level for all species.
Category II
Drugs that require withdrawal at the lowest continuous use level for at least one species for which it is approved or is regulated on a “no residue” or “zero tolerance” basis because of carcinogenic concerns.
There are three types of medicated feeds that can incorporate drugs from categories I or II:
Type A · Medicated Article This product is classified as a drug by the FDA and must be classified as a “Medicated Type A Article” on the label. The manufacturer of a Type A article must hold an effective new animal drug application (FD-356) and comply with the current medicated premix and good manufacturing practice regulations.
Type B · Product This product is classified as a medicated feed. The manufacturer of a Type B product from a Type A article containing a Category II drug requires a medicated feed application (FDA - 1900).
Type C · Product Type C products are intended to be used as a complete feed. The manufacture of a Type C product containing a Category II drug manufactured from a Type A article requires a medicated feed application (FDA - 1900).
What is a nutraceutical?
Unfortunately, no legal definition for “nutraceutical” exists. It is generally assumed that a nutraceutical is compound between a nutrient and pharmaceutical. Although these compounds/ ingredients have a defined nutritional role, pharmacological doses (many fold greater than concentrations needed to elicit a nutritional response) elicit separate effects on pig health or growth. Examples of ingredients considered as nutraceuticals are: zinc oxide, copper sulfate, carnitine, organic chromium, and conjugated linoleic acid. Because nutraceuticals are labeled as dietary supplements, they are regulated under the Federal Food, Drug, and Cosmetic Act (FFDCA).
Compounds not receiving GRAS (Generally Recognized as Safe) status, (i.e., ingredients that do not have a previous history of use in animal feeds) are of concern. Specifically, ingredients that make claims regarding the treatment, prevention, cure, or mitigation of a disease; or affect the structure/ function of the body not related to its nutritional role are considered a drug under FFDCA regulations. Although the FDA has placed lower significance on regulating nutraceutical ingredients without drug claims, the FDA’s condonation of these ingredients is not indicated.
How do feed additives affect pig performance?
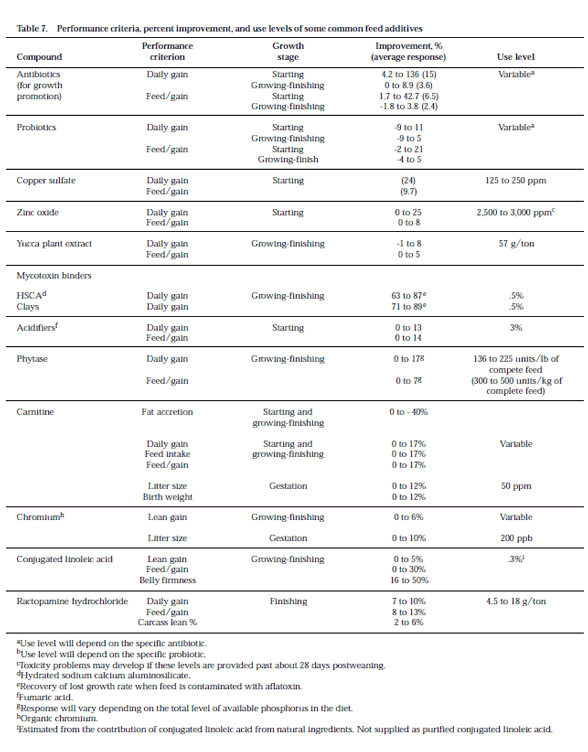
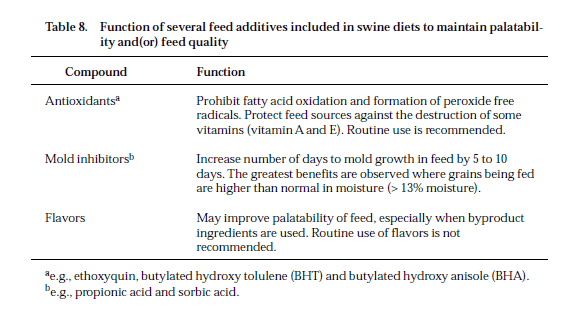
There are many feed additives that have been documented to affect pig performance. Unfortunately, there is not enough space available in this section to cover all these feed additives in detail. The recommended levels for several feed additives are not provided because of either variable usage in the industry or pending status with the FDA. In all cases, if feed additives are to be used, manufacturer and federal guidelines should be followed. Presented in Table 7 is a brief description of the performance criteria, percent improvement, and use levels for several feed additives. For most of these feed additives, responses have been identified within a range to indicate the variability reported in the literature. The response to antibiotics varies considerably due to age of the pig, disease level, type and level of antibiotic, season of year, and other environmental factors. Younger pigs show a greater response than older pigs to antibiotics in the feed (Table 7). In most cases, these responses were recorded in “clean” environments (i.e., the overall health status of the pigs and housing conditions were good to excellent). In a “dirty” environment, the response to antibiotics may be greater than shown in Table 7. Antibiotics do not substitute for good management, especially a thorough cleaning of facilities. It may be more economical to correct the underlying problem affecting pig performance than to use antibiotics in the feed. Some compounds are included in swine diets to avoid feed spoilage and promote feed intake (Table 8). Although improvements in performance are not cited for the feed additives listed in Table 8, circumstances exist where their inclusion in swine diets may increase feed intake and hence gain.
How do I choose a feed additive?
The information in Tables 7 and 8 is presented to allow one to estimate the economic benefit of using some feed additives. When an improvement in feed efficiency is shown, use that to estimate the economic benefit of using the additive. For example, assume feed/gain is improved by an average of 2% when an antibiotic is added to finisher feed. If feed without an antibiotic costs $100/ton, you can afford to pay about $102/ton (100 × 1.02) for the medicated feed assuming no benefit from improved daily gain. If a faster growth rate is considered important, factor that in also. When considering a feed additive, give high priority to feed additives that show consistent results from research trials. Also, consult the feed label to learn what the additive is approved for and withdrawal time. Feed additives increase the cost of the diet, thus it is important to reevaluate their use periodically.
How much antibiotic can be added to feed and can antibiotics be mixed together?
Consult the feed label or the Feed Additive Compendium for details on the approved level(s) in complete feed and which antibiotics can be fed in combination. If it is not legal to feed certain antibiotics together, consider feeding them in rotation. Moreover, rotating antibiotics may be useful if there is evidence that the effectiveness of the current antibiotic is decreasing. The rotation may be annual or when pigs are switched to different diets.
What are the withdrawal periods for feed additives?
Certain feed additives must be withdrawn from the feed before slaughter to ensure residue-free carcasses. Consult the feed label for withdrawal time for the specific feed additive that is being fed.
Should antibiotics be fed to the breeding herd?
Herds that have experienced problems with conception rates and numbers of pigs born and weaned have often been helped by the addition of therapeutic levels of antibiotics to sow diets. Antibiotics are effective if fed for two weeks before and after breeding and(or) from one week before farrowing to weaning. Results from a regional research study (850 litters) showed an improvement in litter size (.5 pigs/ litter) and a slight reduction (negative response) in feed intake during lactation with the addition of chlortetracycline (200 g/ton) from one week before to 15 days after breeding. In the same study, chlortetracycline addition from day 110 of gestation to weaning improved the overall conception rate nearly 6%. In instances where reproductive problems prevail in a herd, a specific diagnosis should be made in consultation with a veterinarian or swine specialist prior to routine inclusion of antibiotics in breeding herd diets. Check the withdrawal time to avoid carcass residues in cull sows.
How do probiotics and antibiotics differ?
Probiotics play a different role than antibiotics in the digestive tract. It has been theorized that probiotics increase the population of desirable microorganisms instead of killing or inhibiting undesirable organisms. The most common microorganisms included in probiotic products are Lactobacillus species, which are normal inhabitants of the digestive tract of healthy animals. These bacteria may help remove waste products and inhibit the growth of certain undesirable bacteria. The response to probiotics in pig feed appears to be greater for starting compared to growingfinishing pigs (Table 7). When positive responses have been observed with probiotics it has usually been at weaning.
What is carnitine and its effect as a feed additive?
Carnitine is a naturally occurring nutrient and until now was widely thought to be synthesized in sufficient quantities by the pig to meet its requirement. It is involved in the transport of fatty acids into certain parts of the cell so they can be used to produce energy. Carnitine has received limited attention in the growing-finishing phase of production. Although improvements in feed efficiency and leanness have been observed, recent reports have only documented the response in early-weaned pigs (until 35 days postweaning). There have been interesting findings from experiments examining the role of carnitine in gestation diets. Studies indicate that inclusion of 50 ppm carnitine in gestation diets can improve litter size and(or) pig birth weight. It appears that the improvements in litter size and birth weight may be related to the duration of carnitine supplementation. Nonetheless, the response of both litter size and birth weight have ranged from 0 to 12%.
What effect does chromium have as a feed additive?
Chromium (Cr), specifically organic Cr (Cr 3+) has been identified as having a role in swine feeding programs. It should be kept in mind that although pigs do have a Cr requirement and Cr is found in pig tissues, forms of elemental Cr, e.g., Cr 6+ can be toxic. Thus, the role that organic Cr fulfills may be in addition to its classical nutrient role - see previous section on nutraceuticals. Organic Cr (namely, Crtripicolinate) has been shown to improve growing-finishing growth performance, and carcass leanness. The results have been variable and some researchers have failed to detect improvements in carcass characteristics or growth performance criteria. Recently, favorable responses in sow reproductive performance have been observed with the addition of organic Cr to sow gestation and lactation diets. Supplementation of 200 ppb of Cr from Cr-tripicolinate improved sow fertility, number of pigs born and weaned, and reduced sow death loss. Females need to be fed the Cr for about six months before an improvement in reproductive performance can be expected.
What is conjugated linoleic acid and its effect as a feed additive?
Conjugated linoleic acid (CLA) is a mixture of polyunsaturated fatty acids. CLA is found primarily in products derived from ruminants. It is produced by the micoflora in the rumen and can be purchased commercially. The most consistent and dramatic effect of including CLA in the diet of growing-finishing pigs has been on belly firmness, and to a lesser degree on feed efficiency and carcass lean percentage (see Table 7).
What is ractopamine hydrochloride and its effect as a feed additive?
Ractopamine hydrochloride is a synthetic beta-adrenergic agonist. Ractopamine has a chemical structure similar to dopamine, norepinephrine, and epinephrine, which are naturally occurring substances in animals. These substances, including ractopamine, affect body metabolism via adrenergic receptors on specific tissues. Ractopamine is approved for increased rate of weight gain, improved feed efficiency, and increased carcass leanness in finishing swine fed a complete ration containing at least 16% crude protein from 150 to 240 lb. A feed mill or veterinary feed directive are not required for use of ractopamine. Because ractopamine lowers feed intake 2 to 4% and increases carcass muscle deposition, logic dictates that the dietary amino acid requirement on a percentage basis and possibly on an absolute (total) basis is increased.
This article hasn't been commented yet.


Write a comment
* = required field